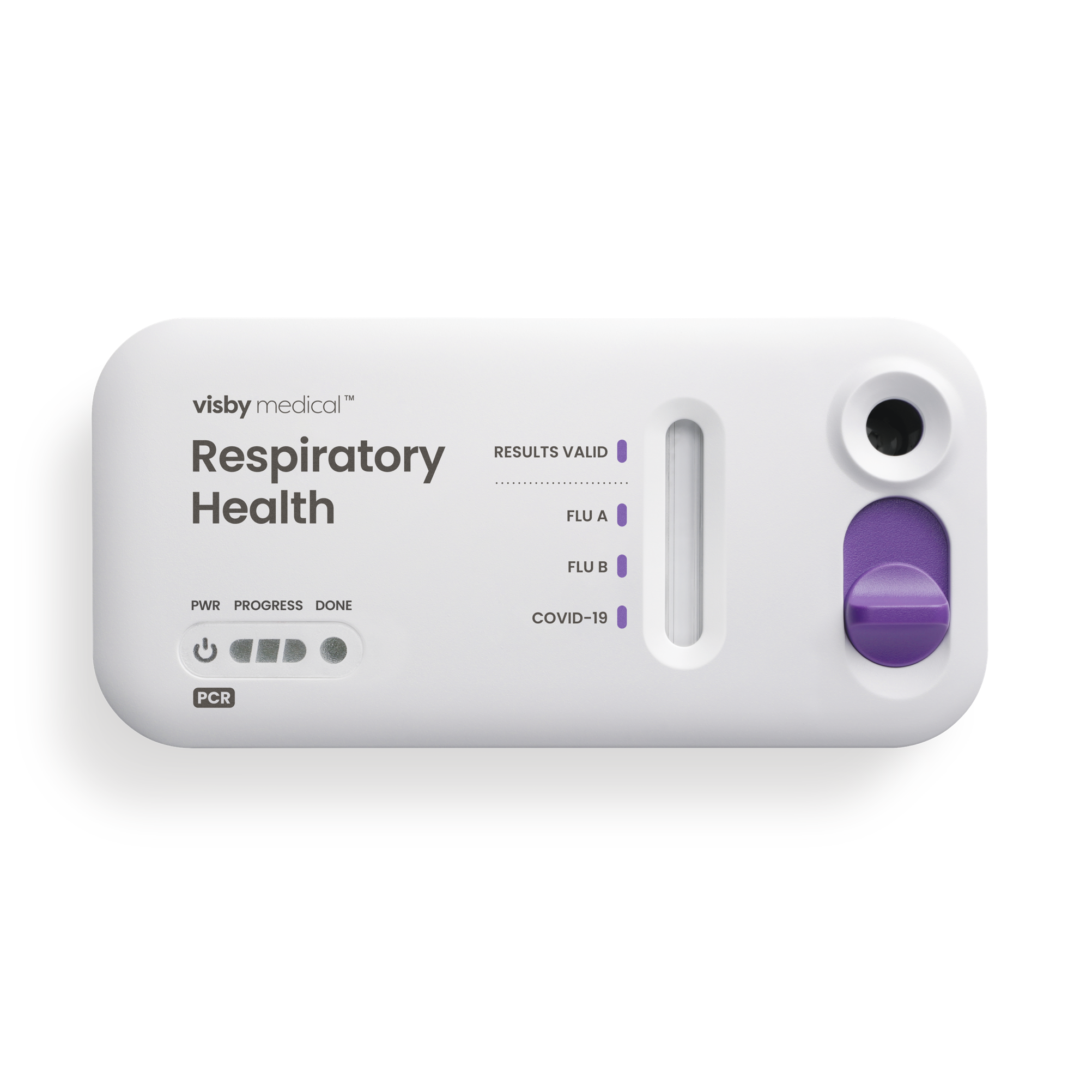
Specifications
CLIA Waived
Test type: PCR (integrated disposable PCR cassette)
Run time: 30 minutes
Collection type: Nasopharyngeal or lower nasal swab
For use with sterile nylon, foam, or polyester flocked swabs.
SARS-CoV-2: 97.4% PPA, 98.8% NPA
Flu A: 97.9% PPA, 99.3% NPA
Flu B: 100.0% PPA, 100.0% NPA
CPT Code: 87636-QW
Manufacturer: Visby Medical
Storage & Expiration
Store at 2-30°C (35-86°F)
Do not freeze any contents of the kit.
Reagents and materials are stable until expiration date printed on outer packaging.
Test device must remain in the sealed pouch until use.
Box Components
Test Devices (20)
Transfer Pipettes (20)
Buffer Tubes (20)
Disposal Bags (20)
Manuals & Documents
Intended use
The Visby Medical Respiratory Health Test is a single-use, instrument-free molecular in vitro diagnostic test utilizing reverse transcription polymerase chain reaction (RT-PCR) technology for the qualitative detection and differentiation of nucleic acid from influenza A, influenza B, and SARS-CoV-2. It is designed for use with clinician-collected nasopharyngeal and anterior nasal swab specimens, or clinician-instructed self-collected nasal swabs (collected on-site) from individuals showing symptoms of respiratory tract infection consistent with COVID-19. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform high, moderate, or waived complexity tests. The Visby Medical Respiratory Health Test is authorized for use at the Point of Care (POC), in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.