Empowering Urgent Care Clinics and Physician Offices with Rapid STI Testing Solutions
In today’s fast-paced healthcare environment, providing timely and accurate testing for sexually transmitted infections (STIs) is crucial for patient care. For urgent care clinics and physician offices, the ability to diagnose and treat common STIs such as Chlamydia, Gonorrhea, and Trichomoniasis in a single visit can significantly improve patient outcomes and satisfaction. With rising infection rates, particularly among women and younger populations, it’s essential that healthcare providers offer quick, reliable, and easy-to-administer STI tests.
The Visby Medical PCR point-of-care device is a game-changing tool designed to meet these needs. By delivering rapid results in under 30 minutes with ~97% accuracy, this device is empowering clinics to test, diagnose, and treat patients in a single visit, eliminating the delays and uncertainties that often accompany traditional lab testing.
The Growing Need for Point-of-Care STI Testing
According to the Centers for Disease Control and Prevention (CDC), STIs are a growing concern in the United States, with millions of new infections reported annually. In New York City, recent data shows alarming spikes in STI rates, especially among women aged 15 to 24, who are five times more likely to contract Chlamydia than any other age group. Left untreated, Chlamydia can lead to pelvic inflammatory disease (PID), infertility, and other serious reproductive health issues.
For urgent care clinics and physician offices, early detection is key. However, traditional lab testing can take days, causing delays in treatment and leaving patients in limbo. With the Visby Medical PCR device, healthcare providers can offer accurate STI test results during the patient’s visit, enabling immediate treatment and reducing the need for follow-up appointments.
The Visby Medical PCR Device: A Breakthrough for Clinics
The Visby Medical PCR device is revolutionizing STI testing by offering rapid, accurate results at the point of care. Designed with busy clinics and physician offices in mind, this CLIA-waived device is easy to use, requiring no additional instruments or laboratory setup. With the ability to diagnose three of the most common STIs—Chlamydia, Gonorrhea, and Trichomoniasis—in less than 30 minutes, the Visby device empowers healthcare providers to deliver faster, more effective care.
Key benefits of the Visby Medical PCR device for urgent care clinics and physician offices include:
Rapid Results: With STI results available in under 30 minutes, healthcare providers can test, counsel, and treat patients in a single visit, eliminating the need for follow-up calls or appointments.
High Accuracy: The device delivers ~97% accuracy for detecting Chlamydia, Gonorrhea, and Trichomoniasis, giving providers the confidence to make informed treatment decisions.
CLIA-Waived: This device is simple to operate and does not require additional lab equipment, making it ideal for urgent care clinics and physician offices that want to offer STI testing without the complexities of lab management.
Antibiotic Stewardship: By providing accurate diagnoses, the Visby device helps prevent the over-prescription of antibiotics, ensuring that only patients with confirmed infections receive treatment.
Why Rapid STI Testing Matters for Your Patients
For many patients, the experience of visiting an urgent care clinic or physician’s office for STI testing can be stressful. Traditionally, they must wait days for test results, prolonging anxiety and delaying necessary treatment. With the Visby Medical PCR device, patients can leave the clinic with their results in hand, allowing for immediate conversations about treatment options and sexual health.
The ability to provide same-day results is particularly important for patients who are asymptomatic, which is common with infections like Chlamydia and Trichomoniasis. The CDC estimates that in 2018 alone, more than 4 million Chlamydia infections occurred, yet many of these cases were undiagnosed because patients did not experience symptoms. By offering rapid, in-office testing, clinics can catch these silent infections early, preventing long-term complications and improving public health outcomes.
Targeting Three Critical STIs
The Visby Medical PCR device focuses on three of the most common and treatable STIs: Chlamydia, Gonorrhea, and Trichomoniasis. These infections, if left untreated, can lead to severe health complications, particularly for women.
1. Chlamydia
Chlamydia is the most commonly reported bacterial STI in the U.S., affecting millions of Americans each year. Untreated Chlamydia can lead to PID, which can cause infertility and chronic pelvic pain. The Visby device allows for the rapid detection of Chlamydia, enabling healthcare providers to treat patients before complications arise.
2. Gonorrhea
Gonorrhea, another bacterial infection, poses a serious risk to reproductive health if untreated. It can lead to infertility and, in pregnant women, it can cause infections in newborns during childbirth. The Visby device’s ability to quickly and accurately detect Gonorrhea ensures that patients receive timely treatment, reducing the risk of complications.
3. Trichomoniasis
Trichomoniasis is caused by a protozoan parasite and is the third most common STI in the U.S. Often asymptomatic, Trichomoniasis increases the risk of contracting other STIs, including HIV. Early detection through rapid testing with the Visby device is crucial for preventing the spread of this infection and promoting better overall sexual health.
Addressing STI Disparities in Your Community
STIs disproportionately affect underserved communities, where access to healthcare is limited. For instance, in Michigan, recent data highlights a troubling rise in syphilis rates among women and infants. These populations often face barriers to receiving timely care, resulting in higher infection rates and complications. By offering rapid, point-of-care STI testing, urgent care clinics and physician offices can play a pivotal role in reducing these disparities.
The Visby Medical PCR device is especially beneficial for clinics serving communities with limited healthcare resources. Its ease of use and rapid results mean that clinics can provide high-quality care without requiring extensive lab infrastructure. This is critical in addressing the rising rates of STIs in areas where healthcare access is limited or where patients may face difficulties in returning for follow-up visits.
The Bottom Line: A New Standard for STI Testing in Urgent Care
Incorporating the Visby Medical PCR device into your urgent care clinic or physician office can transform the way you approach STI testing and treatment. By providing rapid, accurate results, this device enables healthcare providers to diagnose and treat patients in a single visit, improving patient satisfaction, reducing follow-up calls, and ensuring that infections are treated promptly.
Dr. Sarah Braunstein, assistant commissioner for the New York City Department of Health’s Bureau of Hepatitis, HIV, and Sexually Transmitted Infections, emphasized the importance of STI testing: "Getting tested for STIs is of utmost importance. Knowing your STI status allows you to take care of yourself and your sexual partners, get treatment if you need it, and acts as a gateway into longer-term sexual health care."
For urgent care clinics and physician offices, the Visby Medical PCR device offers a solution that meets the needs of both patients and healthcare providers. By delivering fast, reliable results, this device ensures that patients receive the best possible care without the stress and uncertainty of delayed lab results.
As STI rates continue to rise, especially among women and younger populations, the demand for rapid, point-of-care testing has never been greater. The Visby Medical PCR device represents a new gold standard for urgent care clinics and physician offices, providing an efficient, easy-to-use solution that delivers accurate results in less than 30 minutes.
By offering same-day STI results and treatment, you can enhance patient care, reduce the burden of follow-up appointments, and play a critical role in combating the spread of STIs in your community. Invest in the future of healthcare by incorporating the Visby Medical PCR device into your practice—because when it comes to sexual health, every minute counts.
New Cal/OSHA COVID-19 requirements effective May 2022
Read about Cal/OSHA’s new requirements for all employers in California under the COVID-19 Emergency Temporary Standards (ETS), May 2022 revision.
Cal/OSHA implements and enforces California’s COVID-19 safety regulations for businesses. On November 30, 2020, Cal/OSHA put into effect the first COVID-19 Prevention Emergency Temporary Standards (ETS), and has since issued four revisions during the pandemic:
November 30, 2020 (Original ETS)
June 17, 2021 (First revision)
January 14, 2022 (Second revision)
May 6, 2022 (Third revision)
The latest ETS, now in effect until December 31, 2022, includes several new requirements for all workplaces with two or more people.
Summary of Changes
All businesses must:
Have a written COVID-19 Prevention Program
Train employees on the COVID-19 Prevention Program
Have face masks available to employees at no cost
Have N95 respirators available to employees at no cost
Have testing available to employees at no cost, during work hours, in the following situations:
Employee has COVID-19 symptoms
Employee had a close contact with a person with COVID-19
During an outbreak or major outbreak
Exclude employees that test positive for COVID-19 from the workplace. Employees can return after 5 days if the employee:
Has a negative test
Symptoms are improving
They wear a face covering at work for an additional 5 days
During an Outbreak (three or more COVID-19 cases among employees in an exposed group within a 14-day period), employees who had close contacts must test negative or be excluded from the workplace until the return to work requirements for COVID-19 cases in are met.
During a Major Outbreak (20 or more COVID-19 cases in an "exposed group" within a 30-day period), all employees in the exposed group must test negative or be excluded from the workplace until the return to work requirements for COVID-19 cases in are met.
COVID-19 Prevention Program and Employee Training
The written COVID-19 Prevention Plan must include, among other things:
Establishing communication systems for how employees can participate and how issues and cases are reported and handled.
COVID-19 hazard assessment and inspections
Information about how to obtain face masks and N95 respirators at no cost
Information about hand washing, sanitizing, and cleaning
Information about how to obtain testing at no cost to the employee during work hours
Information about investigating, recording, and reporting COVID-19 cases
How employees are trained and instructed about the plan
Employee training log, including information about exclusion pay and other benefits
COVID-19 case investigation form
Protocols for handling Outbreak and Major Outbreaks
Documentation about vaccination status (optional)
Information about how the program applies to employer-provided housing and transportation (if applicable)
Cal/OSHA ETS Compliance Packages:
Face Masks and N95 Respirators
All organizations must provide face masks and N95 respirators to employees at no cost. Respirators must NIOSH approved. KN95 masks or respirators that are not NIOSH approved do not fulfill the requirement. NIOSH stands for National Institute for Occupational Safety and Health. The agency operates under OSHA and has a testing, approval, and certification program assuring respirators used in the workplace meet certain standards for blocking particles. To ensure a respirator is NIOSH-approved, you can check NIOSH’s Certified Equipment List.
COVID-19 Testing
All employers must now offer free testing to employees during work hours in the following situations:
Employee has COVID-19 symptoms
Employee had a close contact with a person with COVID-19
During an outbreak or major outbreak
To comply with the new testing requirements, an employer could utilize one of the following options:
Self-Administered Over-the-Counter Tests. An employer could supply employees with inexpensive over-the-counter tests and allow them to self-administer and self-report their results provided the results can be verified — this could be satisfied by having the employee submit a date and time stamped image of the result, or by having them use an app to upload the date and time-stamped results. This choice is likely the least expensive option from a test cost and employee time standpoint.
On-Site Testing by Third Party. The employer could hire a third party to come to the workplace, or a location outside of the workplace, to perform testing and/or proctor rapid antigen tests and record results. This may be a good option in the event of an Outbreak or Major Outbreak to ensure that tests are performed properly in order to minimize false negative results that could cause COVID-19 to continue to spread in the workplace.
On-Site Proctoring by Designated Employee. The employer could have a designated employee proctor over-the-counter tests and record results either on-site or at an off-site location.
Off-Site Testing. The employer can pay for an employee to get tested off-site during work hours. The employer would have bear the cost of the test and pay the employee for the time required to take the test. There are still some free testing sites around, but there are fewer every day, and many employees may not be in close proximity to a free testing site. Because the employer has to bear the responsibility of the paying the employee for their time to get the test, even if the test itself is free, the wages that would have to be paid for the time to get the test is likely not the most cost-effective option.
Required and Optional Documentation
In addition to a written COVID-19 Prevention Program, the new ETS requires that employers have the following documentation on hand and available immediately upon request:
COVID-19 Hazard Assessment. Employers must assess areas in the workplace that may increase the risk of transmission of COVID-19 and provide ways to address issues and minimize transmission risk.
COVID-19 Inspection Log. Employers must perform inspections of building ventilation and filtration systems, hand washing and sanitizing facilities and protocols, and the availability and proper use of PPE including mask, respirators, face shields, barriers, and gloves, as applicable.
Training Materials. Presentations and other documents provided to employees during the training, which details information about exclusion pay and other benefits that will be provided or obtained if an employee get COVID-19 from the workplace, or outside the workplace.
Training Log. List of employees (with signatures) that have completed COVID-19 Prevention Program training
Case Investigation Log. Employers must documents investigations in which a COVID-19 case, or suspected case, was present in the workplace, the actions taken, and a list of employees contacted about the potential exposure.
Vaccination Log (optional). A log of the vaccination status of employees may be kept. However, the new ETS regulations pertain to all employees regardless of vaccination status.
We hope this helps you digest the new Cal/OSHA ETS requirements for businesses in California. If you have any questions or would like us to help with any of the areas above, we are just a click or call away. We also have Cal/OSHA ETS Compliance Packages if you would like to offload most of the work on us!
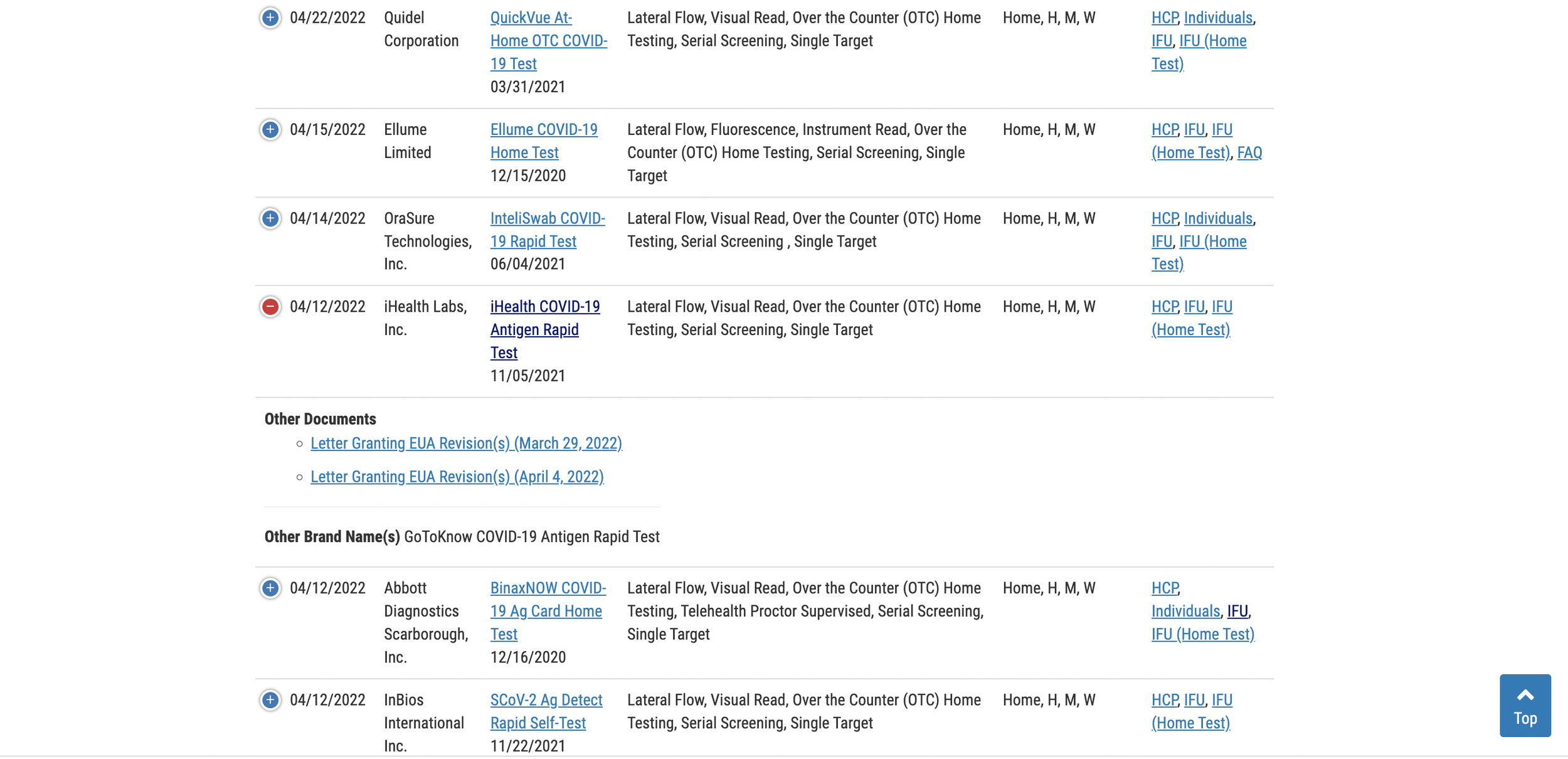
FDA adds expiration date information to rapid test listings
On April 29, 2022, the U.S. Food and Drug Administration updated its list of Emergency Use Authorizations (EUA) of rapid tests to include expiration date extension letters.
On April 29, 2022, the U.S. Food and Drug Administration updated its list of Emergency Use Authorizations (EUA) of rapid tests to include expiration date extension letters.
The updated table for rapid antigen tests can be viewed here.
Scroll down the list to locate your test, then click the (+) to the left of the test name to expand the row and access the expiration date extension documents. If you cannot find your test on the first page, just toggle the list menu to show all listings. By default, the page only displays 25 tests.
Stability of Rapid Tests
Most lateral flow immunoassay test cassettes, like those used in Covid-19 rapid antigen and rapid antibody tests, remain stable for years under normal storage conditions. However, the FDA only allows the manufacturer to print an expiration date on the box that is supported by real-time stability data. Since Covid is a new disease and all tests have been recently developed, most tests will only initially be granted a short 3, 6, or 9 month expiration date.
As manufacturers continue submitting new stability data to the FDA on a rolling basis the tests are granted extensions beyond those printed on the box at time of manufacture. So even if the expiration date printed on your box is coming up soon, or has passed, there’s a very good chance your tests haven’t really expired. Now you can cross-reference the FDA website to see if any extensions were granted. Most Covid rapid tests have not expired yet and will ultimately be granted a final shelf-life of 24 to 36 months.
California Department of Public Health endorses use of rapid tests past FDA expiration dates
The California Department of Public Health went even further than the FDA on March 4, 2022, and endorsed the use of over-the-counter rapid tests even beyond the FDA authorized expiration, indicating that as long as the control lines register properly on the test, it can be used. The extension is valid until further notice.
Here is the CDPH’s full public notice, the image is linked to the original statement posted on its website.
If you have any questions about expiration dates, or would like us to help you find your test’s expiration date with the extensions, just contact us.
Doctors: Should you antibody test?
As the COVID-19 pandemic rolls on, it is important to know what kind of testing should be performed and when it is useful. Otherwise, doctors may later lack important medical information about their patients.
As the COVID-19 pandemic rolls on and increased wide-spread testing becomes available nationwide, antibody tests will become widely available at testing sites, urgent cares, and emergency rooms. Should you provide these tests for your patients ? What information will they provide? Are they worth doing? Here are some things to consider.
First, it is important to understand that antibody (serology) tests are not stand-alone diagnostic tests! If an individual starts having a fever and cough, and runs over to the nearest drive-up testing site that only offers antibody testing, this individual would need to drive away and find a site that offers nasal swabs (molecular tests). With that being said, the viral nasal swab PCR tests, which are the diagnostic “Gold Standard”, may have up to a 20-50% false negative rate. This means that 1 in 5 individuals who truly have the disease are not caught by these nasal swab tests, according to a Johns Hopkins meta-analysis.
If an antibody test with high sensitivity is run concurrently with a viral test, a positive IgM/IgG antibody test would suggest a recent onset coronavirus infection, and can be positive as early as a day post symptom onset in some patients. This may provide for greater confidence that a negative nasal swab test result is truly negative versus a false negative, even in the acute phase of disease, helping to identify those individuals with disease whom otherwise would think they are virus-free and continue to spread disease.
Even antibody tests with the highest sensitivity would miss some patients who have disease as antibody titers within the first week may be too low to detect with point-of care antibody tests in some patients, but not all. A clinician should use their best judgment based on the patient medical history and physical exam to determine, in the acute setting, whether or not they need to draw any blood for labs, including a serology test.
If a patient is greater than seven days out from symptom onset, it is important to know that at this point, the accuracy of nasal swabs has declined even more, and that the amount of antibodies have increased in the blood, so it would be important to administer both the nasal swab and an antibody test. If a patient is more than two weeks out from symptom onset, in most patients an antibody test alone would allow you to determine whether they have had COVID-19 except for in those who are immunocompromised or have an immune disorder. Administering a nasal swab test will have a very high rate of false negatives, much higher than an antibody test.
Antibody tests also help to identify those who are asymptomatic, as well as those who are symptomatic but never get tested due to mild symptoms. A recent epidemiological study from Spain showed that 1/3 of the greater than 61,000 cohort in their study were asymptomatic and almost 20% of those who were symptomatic never received testing. Seroprevalence studies here in the U.S. have similar findings with one study in California showing that the reported number of cases is as much as 55-fold lower than the actual number of infections.
Why is this important? In order to understand how deadly this new disease is, the infectious fatality rate (IFR) must be known. That is, the proportion of infected people who will die as a result of this disease, including those who don't get tested due to mild or no symptoms. If those detected are a smaller proportion than those actually infected, our infectious mortality rate will be much higher than truly expected. This would give false implications of the scale of this pandemic and skew the responses of governments and individuals towards overreaction nationwide, which could cause tremendous physical and mental harms from the overuse of public health interventions like lockdowns and social distancing, as well as cause extreme financial and economic strain. On the other hand if the IFR is calculated too low, this could lead governments and individuals to under-react, causing increased loss of lives and overburdening of our hospitals and hospital staff. It is important to get the IFR right within your specific region and amongst varying demographics.
Finally, how will we truly understand the future relationship between SARS-CoV-2 antibodies and immunity from COVID-19 reinfection if we miss the majority of those infected initially? How likely is COVID-19 reinfection for people with and without detectable SARS-CoV-2 antibodies? This will be hard to accurately assess with many individuals remaining undetected for both disease and antibodies. As this pandemic rolls into later parts of the year, will a COVID-19 infection be a primary infection or a reinfection? If there are individuals truly being re-infected, what does this tell us about the possible efficacy of vaccinations? We don't have enough information from scientific studies to provide the answers to these questions, but once you know who has had COVID-19 within your patient population, you will be able to also know later whether it is a reinfection or a primary infection.
If there are enough reporting antibody test results, doctors will have the information they need within their communities, and will be better able to advise, educate, and advocate for their patients, communities, and government leaders. Active and passive surveillance are so important. We cannot wait until the pandemic is over to begin antibody testing or seroprevalence studies. Understanding COVID-19 disease transmission and deadliness is limited if our diagnostic testing is only able to find those with symptoms some of the time, those without symptoms probably even less of the time, and those who have had symptoms and won't get tested, ever. Antibody testing can help with detection within each of these groups.
References:
Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019
Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19)
COVID-19 Antibody Seroprevalence in Santa Clara County, California
Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020
Clinical and Immunological Assessment of Asymptomatic SARS-COV-2 Infections
Contact Us for More Information
If you have any questions or would like more information about antibody testing or the antibody tests we supply, feel free to submit your information below and we’ll reach out to you.
Why the CDC recommends using two different antibody tests.
In its Interim Guidelines for Antibody Testing, the CDC recommends using two different antibody tests to improve accuracy, especially when confirming a positive test result. This article breaks down what sensitivity and specificity actually mean and explains the math behind the CDC’s recommendation.
Read time: 3.5 min.
In its Interim Guidelines for Antibody Testing, the CDC recommends using two different antibody tests to improve accuracy, especially when confirming a positive test result. This article breaks down what sensitivity and specificity actually mean and explains the math behind the CDC’s recommendation.
What do Sensitivity and Specificity mean?
Autobio and Healgen antibody tests. The CDC says two is better than one.
The CDC and FDA urge labs and clinics to perform antibody testing with tests that have high sensitivity and specificity. But what do these terms mean in the real world?
Sensitivity is how good a test is at correctly identifying COVID-19 positive samples. For antibody testing, 100% sensitivity means that a test will detect antibodies in individuals that have been infected with coronavirus 100% of the time. If there are 50 positive samples, it will detect 50 out of 50. In other words, it is highly sensitive. If the test detects 48 out of 50, it would have 96% sensitivity. Another way of saying this is the test will give a false negative result only 4% of the time.
In the real world, no test will give the correct result 100% of the time if enough samples are used, but a really good test can achieve 99% or higher. 96% sensitivity is still very good, but below we’ll explain why it can be problematic if the infection rate is low in a population. The other crucial aspect of antibody testing is that most people don’t immediately develop antibodies to coronavirus. According to a recent clinical study, the average time it took for infected individuals was 13 days. One hundred percent of patients developed antibodies by Day 19. So antibody tests become most sensitive around 2-3 weeks after exposure, and would be much less sensitive during the first week.
Sensitivity is how good a test is at correctly identifying COVID-19 positive samples. 100% sensitivity means if there are 50 positive samples, the test will correctly detect 50 out of 50. If it detects 48 out of 50, that would be 96% sensitivity.
In some clinical studies and Instructions for Use, sensitivity is referred to as “Positive Percent Agreement.” They mean the exact same thing and are both reporting what percent of the time the test correctly identifies samples known to be positive for coronavirus.
Specificity is how good a test is at correctly identifying COVID-19 negative samples. 100% specificity means that a test will correctly detect no antibodies in a person who has never been infected with coronavirus 100% of the time. If there are 50 negative samples, it will detect 50 out of 50. In other words, it is highly specific because it does not detect COVID-19 when a person has not been exposed to coronavirus, or has had a different type of illness, like a cold or flu. If a test correctly identifies 49 out of 50 negative samples, it would have 98% specificity. Another way of saying this is the test will give a false positive result just 2% of the time.
Specificity is how good a test is at correctly identifying COVID-19 negative samples. 100% specificity means that a test will correctly detect a negative patient 100% of the time. If there are 50 negative samples, it will detect 50 out of 50. If it detects 49 out of 50, that would be 98% specificity.
Some clinical studies and Instructions for Use refer to specificity as “Negative Percent Agreement”, but they mean the same thing — reporting what percent of the time the test correctly identifies samples known to be negative for coronavirus. Using tests with a high specificity becomes very important when the infection rate is relatively low in a population, especially under 5%, and is the reason why the CDC recommends using two different antibody tests, which we explain next.
Why should two different antibody tests be used, especially to confirm a positive result?
Let’s use the two antibody tests we carry as an example — Autobio and Healgen. Both are EUA authorized and were clinically validated by the FDA’s serology testing program in conjunction with the National Cancer Institute. In other words, they passed the FDA’s criteria for sensitivity and specificity. So far only four lateral flow assays have been granted EUA.
As reported in the FDA’s test results, Autobio has a Sensitivity of 99.0% and a Specificity of 99.0%. Healgen has a Sensitivity of 100.0% and a Specificity of 97.5%.
This means if 100 individuals known to never have been exposed to coronavirus were tested with Autobio, it would correctly identify 99 out of 100 people, and would only give one false positive result. Fantastic, right?
Well, let’s say that only 5% of the population has been infected with coronaviurs. That means Autobio would detect the 5 patients out of 100 that have been infected, but would also give that 1 false positive result. That means it would only correctly detect 5 out of the 6 people who were actually exposed to coronavirus, meaning the test it is correct about 85% of the time. This is called the Positive Predictive Value, or PPV. So even 99.0% specificity means that about 15% of patients will be given a false positive result.
PPV depends on the percentage of the total population that has been infected. If 20% of the population had been infected, then the PPV would increase to around 95%, so the test becomes more reliable. But there is another solution.
The predictive value of antibody testing increases greatly if two really good tests are used together, especially when the infection rates are relatively low in a population.
Using two tests, called “orthogonal testing”, each with high sensitivity and specificity greatly increases the predictive value so long as the two tests were independently developed and have different formulations. Optimally, you would choose a test that targets two different antigens. In the case of coronavirus, one that targets the spike protein, and one that targets the nucleocapsid protein would be ideal. But even using two tests that both target the spike protein is valuable as long as the tests were independently developed because they likely target and detect the spike protein in slightly different ways.
Using Autobio and Healgen together increases the PPV to around 99.5%, which means if both tests give a positive result, there is a 99.5% probability it is the correct result. Performing orthogonal testing greatly increases the predictive value of the test, especially when infection rates are relatively low in a population.
The combined Negative Predictive Value of Autobio and Healgen, or NPV, is 99.9%. This means that if both tests return a negative result, there is a 99.9% probability it is the correct result.
You can download the CDC’s PPV calculator here and enter the sensitivity and specificity of two tests and get the PPV and NPV back.
We hope this has helped you understand why using two antibody tests is ideal. If you have any questions, or are interested in setting up testing at your lab or clinic, we would be happy to provide more information or supply your test kits. Just reach out and let us know how we can help.
How should nursing homes conduct COVID-19 testing?
Nursing homes are required to submit weekly COVID-19 reports to remain compliant with new Center for Medicare and Medicaid Services regulations. The CDC has created a training course and issued guidelines for performing testing in nursing homes. Here is a summary.
It is estimated that between one-third and one-half of all COVID-19 deaths in the United States have occurred in nursing homes. Organizations providing care and housing for the elderly should perform regular testing of all residents and staff. The CDC has issued guidelines for COVID-19 testing in nursing homes and just updated and expanded those guidelines on June 25, 2020. All the appropriate links can be found at the bottom of this article. We encourage you to read them for yourself, but we have done our best to break down CDC and CMS’s wealth of information and guidelines into a form that is easier to understand.
This article is not intended to serve as the sole source of information from which to base decisions regarding care and testing. Consult other sources of information, including physicians, lawyers, and other professionals who are familiar with your facility and can offer more specific, customized advice for your community. This article will focus primarily on testing, not infection prevention and control methods as recommended by the CDC.
Step 1.
Appoint a person, or group of people, to be in charge of managing the crisis.
The person(s) appointed for managing the COVID-19 crisis will be responsible for developing infection prevention and control policies and procedures, performing infection surveillance, providing training of staff, and ensuring adherence to policies. This should be a full-time position for at least one person in facilities with more than 100 residents or that provide ventilator or dialysis services. Smaller facilities should still consider appointing a full-time position if resources allow.
The appointed person(s) should complete the CDC’s online Nursing Home Infection Preventionist Training Course.
When training has been completed, the CDC recommends you add one of their training course badges to your website to help reassure families and prospective clients that you are taking action to make your community safe and healthy during the crisis. Code snippets for the banners can be found on the CDC’s website here.
Step 2.
Comply with CMS reporting requirements by submitting weekly reports.
On May 8, 2020, the Center for Medicare and Medicaid Services (CMS) mandated certain COVID-19 reporting requirements including:
Suspected and confirmed COVID-19 infections among residents and staff, including residents previously treated for COVID-19
Total deaths and COVID-19 deaths among residents and staff
Personal protective equipment and hand hygiene supplies in the facility
Ventilator capacity and supplies in the facility
Resident beds and census
Access to COVID-19 testing while the resident is in the facility
Staffing shortages
Other information specified by the Secretary
Facilities must submit the data through the National Healthcare Safety Network (NHSN) COVID-19 Module at least once every seven days. All instructions for sign up, use, and reporting is included in the link above. We recommended the person(s) appointed to be responsible for managing the facility’s COVID-19 crisis be put in charge of completing this weekly reporting.
Step 3.
Create and follow through with a swab testing plan.
The CDC recommends that all residents and staff be tested on a regular basis. Testing intervals depend on how recently the last COVID-19 infection was identified. Swab (viral) testing should be the main testing method used because it identifies individuals with active infections during the first week of symptoms. Because antibodies can take as long as 2-3 weeks to develop after symptoms appear, they should not be used to diagnose COVID-19, but they can be used to supplement diagnosis, especially in cases where COVID-19 is suspected but the swab test gave a negative result. Below are our general recommendations for a testing plan. Specifics should be decided by physicians and other experts familiar with your facility’s residents, staff, and operations.
1. Establish COVID-19 Positive, Negative, and Suspected or Exposed Cohorts.
Positive.
The CDC recommends establishing a separate floor, wing, or cluster of rooms for COVID-19 positive residents and that staff be dedicated to only work with residents in this cohort and not be allowed to cross-provide care for residents in other cohorts. Residents in the Positive cohort can be moved back to the Negative cohort after 14 days of no symptoms or positive tests.
Suspected or Exposed.
If residents are suspected of COVID-19, such as when they are exhibiting symptoms, but are still awaiting test results, they should either be moved to a dedicated cohort, or should remain quarantined until test results are returned or illness disappears. After possible exposure to another resident with a confirmed case, exposed residents should be tested every 3-5 days even if asymptomatic. Residents can be moved back to the Negative cohort, or un-quarantined, after 14 days of no symptoms or positive tests. Immediately move positive residents to the Positive cohort.
Negative.
The same careful monitoring should be done for the Negative cohort. If a resident exhibits symptoms, tests positive during one of the regularly scheduled site-wide tests, or was exposed to a confirmed Positive case, they should be immediately moved to the appropriate cohort.
2. Perform an initial survey by testing all residents and staff.
Partner up with a lab that is qualified to perform COVID-19 viral testing. They must be CLIA-certified for high complexity testing and must have certain PCR instruments to run the tests. If you need help finding a qualified, equipped lab and ensuring they have enough testing supplies, contact us and we will help you. We supply some of the best swab and antibody tests on the market, all FDA authorized.
The CDC recommends testing all residents and staff to obtain an initial survey of infected community members. Move any residents that tested positive to the Positive cohort. Staff that tested positive should not return to work until their fever is gone and they have two negative tests taken at least 24 hours apart, regardless of whether they had symptoms or not. Any residents exposed to positive individuals should be moved to the Suspected or Exposed cohort or should remain isolated in their room for 14 days, monitored carefully, and tested regularly.
If a resident or staff declines or cannot be tested, take appropriate safety measures to ensure they do not put others in the community at risk.
3. Perform regular testing if positive individuals are identified.
If a positive individual is identified during a round of testing, move them to the Positive cohort, carefully monitor all exposed individuals and immediately test anyone who develops symptoms consistent with COVID-19. Regularly test all residents and staff every 3-5 days, if testing supplies are available, until no new cases are identified for 14 consecutive days. If testing capacity is limited, it is recommended to prioritize:
(a) Residents and staff who were exposed to positive individuals
(b) Residents and staff who were in closest proximity to the positive individuals
(c) Residents who regularly travel to and from the facility for dialysis and other medical treatments
(d) Staff who work at other facilities with known positive cases.
4. If no new positive cases are identified, testing intervals may be increased.
Continue regular, frequent testing of all residents and staff until no new cases are discovered in a round of testing. Then the testing interval may be increased to every 7 days, and then to 14 days. Because we don’t have definitive information about immunity, at this time the CDC recommends testing all residents and staff every two weeks at minimum, even if no new positive cases are detected.
Step 4.
Perform antibody testing to obtain information about your community’s infection rate.
The CDC has stated that nearly all people develop antibodies to coronavirus within 1-3 weeks after symptoms appear, infectiousness is likely greatly reduced in people who produce antibodies, and some degree of immunity is developed. These statements are based on recent clinical studies. However, because it is not known how strong immunity is or how long it lasts, the CDC does not recommend using antibody tests to make decisions about grouping residents together in nursing homes.
The CDC has indicated that antibody tests can be used to determine the spread of coronavirus within a population. We recommend performing a round of antibody testing on all your residents and staff to get an idea of how many people in your community have been infected by COVID-19. While antibody testing should not be used as the sole piece of information to inform resident grouping, we believe the information obtained from a round of antibody testing would be valuable in making more informed decisions about how to manage the crisis in your community.
When performing antibody testing, if a person tests positive, they should be tested with another, different antibody test to confirm the result. This is called “orthogonal testing” and is recommended by the CDC to improve the accuracy and predictive value of test results. If only one test is performed, it can only be assured the test gave the correct result around 60-90% of the time depending on the sensitivity and specificity of the test. However, if two tests give a positive result, that number can jump to over 95%. When used jointly, the two antibody tests we supply have a combined predictive value of 99.7%, meaning that if both tests return a positive result, it is likely the correct result.
The CDC has also stated that antibody testing can be used to supplement diagnosis using swab tests, especially if the swab test is done more than 7 days after symptoms appeared. A recent study showed that using antibody tests jointly with swab tests improved the accuracy of diagnosis during all stages of infection, early and late. So if resources are available, labs are encouraged to run both types of tests.
Help Me Perform Testing
If you have any questions or would like us to help you perform testing, please contact us using the form below. We have helped homes coordinate testing with partner labs and supply their partner labs with the tests and supplies they need.
Important Links
CMS: Memorandum regarding stages of reopening nursing homes
CDC: Responding to Coronavirus in Nursing Homes
CDC: Performing Facility-Wide Testing in Nursing Homes
CDC Testing Guidance for Nursing Homes
CDC: Key Strategies to Prepare for COVID-19 in Long-Term Care Facilities
CDC: Interim Guidelines for Antibody Testing
CDC: Infection and Prevention Control Recommendations in Nursing Homes
CDC: Nursing Homes Infection Prevention Course
What qualifications and instruments do I need to perform antibody testing?
There are different types of COVID-19 antibody tests, each requiring different qualifications and instruments to run. If your clinic, hospital, or lab is considering performing its own antibody testing, here is a breakdown of the types of tests, qualifications, and instruments required to run them so you can make the right decision.
Read time: 3 minutes
There are different types of COVID-19 antibody tests, each requiring different qualifications and instruments to run. If your clinic, hospital, or lab is considering performing its own antibody testing, here is a breakdown of the types of tests, qualifications, and instruments required to run them so you can make the right decision for your organization.
Levels of Laboratory Certifications
All laboratories that perform diagnostic testing on human samples must be registered and certified under the Clinical Laboratory Improvement Amendments of 1988, a set of federal regulations commonly know as CLIA. Some tests require more care and skill to perform and can only be run by laboratories with highly-trained personnel. Other tests are relatively simple to perform and can be run by less-skilled individuals. Each diagnostic test is given a clearance level by the FDA ranging from Waiver to High Complexity. Here is a breakdown of the different levels of certification as they relate to various COVID-19 tests.
1. Waiver
Waiver is the lowest level of clearance required to perform a test. Most clinics apply for and receive this level of clearance. There are currently 5 tests that have been granted waiver status by the FDA. They require a sample to be collected by a swab and then placed in a specialized instrument for analysis. Turnaround times typically range from 30 minutes to a few hours. Some of these tests have not demonstrated as high of sensitivity or specificity as other diagnostic tests, but are much easier to perform because they do not require complicated laboratory processing.
We expect to see more tests granted waiver status over time, including antibody tests. Currently, there are no antibody tests granted Waiver status by the FDA.
2. Microscopy
This certification primarily applies to laboratories that perform microscopy on patient samples — i.e. looking at a sample under a microscope. This does not apply to COVID-19 testing.
2. Moderate Complexity
Moderate complexity tests require a higher level of skill and training to perform correctly. They must be run under the direction of an MD or PhD with clinical laboratory training and laboratory technicians and other staff must meet certain education and training requirements.
There are about 25 tests that have been granted authorization to be performed by moderate complexity labs. These include about 15 antibody tests and 10 swab tests. Some antibody tests still require specialized analyzers to obtain results, as do the swab tests, but they are generally easier to perform than the “gold standard” diagnostic swab tests, which are classified as high complexity.
3. High Complexity
High complexity tests require the highest level of skill and training to perform correctly. They must be run under the direction of an MD or PhD with extensive clinical laboratory training. Laboratory technicians and other staff must also meet more stringent education and training requirements to perform testing at this level.
There are about 80 tests that have been granted authorization for processing by high-complexity labs. These are the vast majority of the “gold standard” swab tests being performed throughout the country. Swab samples are collected by a qualified healthcare provider, placed in a tube with a stabilizing media, and transported on ice to a high-complexity CLIA lab for processing.
There are also nearly 200 antibody tests that are listed on the FDA’s notice to distribute list. These tests do not have EUA authorization and must be run in high complexity labs by default. That list can be found here under the dropdown entitled “Q: What commercial manufacturers are distributing serology test kits under the policy outlined in Section IV.D of the Policy for Coronavirus Disease-2019 Tests?”
Applied Biosystems 7500 Real-Time PCR System used to run some COVID-19 diagnostic tests. Photo credit: Wikimedia Commons
Processing of the gold standard diagnostic tests takes place in a controlled environment to minimize sample contamination and cross-contamination, involves the use of extraction reagents to purify RNA from the samples, and then processing of the samples by a PCR instrument that utilizes thermo-cycling and fluorescence analysis to amplify and detect the genetic material of the virus. Total processing time is around 3 hours. Though there is one authorized PCR test can be run in about 30 minutes.
Types of Antibody Tests
Not all antibody tests are the same. In fact, tests vary widely in required skill levels required to perform and detection methods used. Some tests can be performed in moderate complexity labs while others must be performed in high complexity labs. Some tests require special instruments to obtain results, and others give a simple visual readout, like a pregnancy test. The advantage of more complicated tests is that they usually give more accurate results. However, that is not always the case. Here is a breakdown of the four main types of antibody tests current authorized by the FDA.
1. Lateral Flow Assay
Lateral flow assays are the simplest type of antibody test. They require a drop of blood and a couple drops of buffer to be placed onto a test cassette, followed by a 10-15 minute wait, and then visually reading the results window. The presence or absence of certain test lines determine a positive or negative result. There are currently 4 authorized lateral flow tests. We supply two of them.
2. Chemiluminescence Assay
Chemiluminescence immunoassays are similar to lateral flow assays except they require a special instrument to detect the chemiluminescence. There are currently 9 authorized chemiluminescence tests. Most can be run by moderate complexity labs.
3. Fluorescence Assay
Fluorescence immunoassays are similar to chemiluminescence tests except they detect fluorescence instead of chemiluminescence, which also requires a special instrument. There is currently 1 authorized fluorescence test. Most can be run by moderate complexity labs.
4. Enzyme-Linked Immunosorbent Assay (ELISA)
ELISAs are the most complicated type of antibody test to run. They require high complexity certification, careful preparation of samples, special instruments, and several hours to obtain results. There are currently 5 authorized ELISA tests.
What are the differences in accuracy of the tests?
According to the FDA’s serology test validation program results, on average, ELISA and chemiluminescent assays, which require special analyzers, outperformed lateral flow assays by about 0.5% sensitivity and specificity. However, some of the lateral flow assays performed as well or better than some of the ELISAs and chemiluminescence assays. All of the EUA authorized antibody tests are within about 1% accuracy of each other.
The advantage of the lateral flow assays are that they require no special equipment or training and are likely to eventually obtain Waiver status, which means they can be run at point-of-care by most healthcare personnel. They are also the least expensive.
Conclusion
If you are a moderate complexity lab without special analyzers, a highly accurate lateral flow assay is your best option. If you have an analyzer, or high complexity certification, and would like to improve accuracy, you should consider chemiluminescence or ELISAs. However, check the sensitivity and specificity clinical validation data because there are some exceptional lateral flow assays that outperform more complicated tests.
If you have any questions or would like help setting up antibody testing at your lab, clinic, or other organization, please let us know.