Indicaid Covid-19 Antigen Test
Tests for Covid-19
CLIA Waived
Results in 20 minutes
Lower nasal swab
88.9% PPA, 96.8% NPA
Long-dated expiration
Tests for Covid-19
CLIA Waived
Results in 20 minutes
Lower nasal swab
88.9% PPA, 96.8% NPA
Long-dated expiration
Tests for Covid-19
CLIA Waived
Results in 20 minutes
Lower nasal swab
88.9% PPA, 96.8% NPA
Long-dated expiration
Specifications
CLIA Waived
Test type: Rapid Antigen
Run time: 20 minutes
Collection type: Lower nasal swab
SARS-CoV-2: 89.1% PPA, 99.8% NPA
CPT Codes: Covid-19 87811QW
Manufacturer: Phase Scientific.
Storage & Expiration
Store at 2-30°C (35-86°F)
Do not freeze any contents of the kit.
Reagents and materials are stable until expiration date printed on outer packaging.
Test device must remain in the sealed pouch until use.
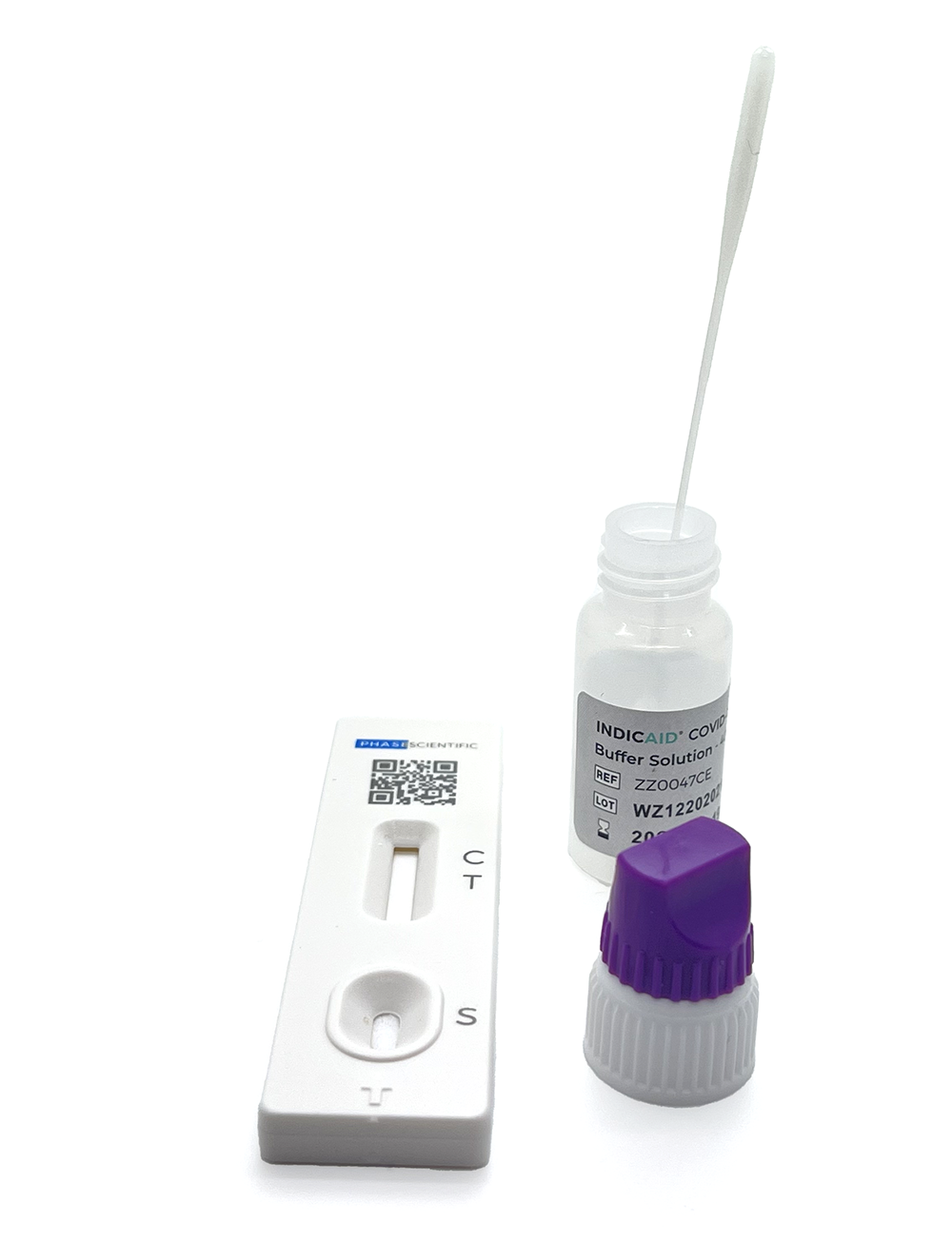
Box Components
Lower nasal swabs (25)
Test cassettes (25)
Extraction buffer tubes (25)
Instructions for use (1)
Quick Reference Guide (1)
Manuals & Documents
Intended Use
The INDICAID COVID-19 Rapid Antigen Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal swab specimens from individuals who are suspected of COVID-19 by their healthcare provider within the first five (5) days of symptom onset. Anterior nasal swab specimens may be collected by a healthcare provider (HCP) or self-collected (by individuals 18 years of age or older, under the supervision of an HCP). Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate complexity, high complexity, or waived tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.